Already, 2023 is looking like a dynamic year for biotech on the state level as state legislatures nationwide get ready to tackle a few key issues for the industry.
Here are the top four big-ticket items for biotech to watch in state policy in 2023.
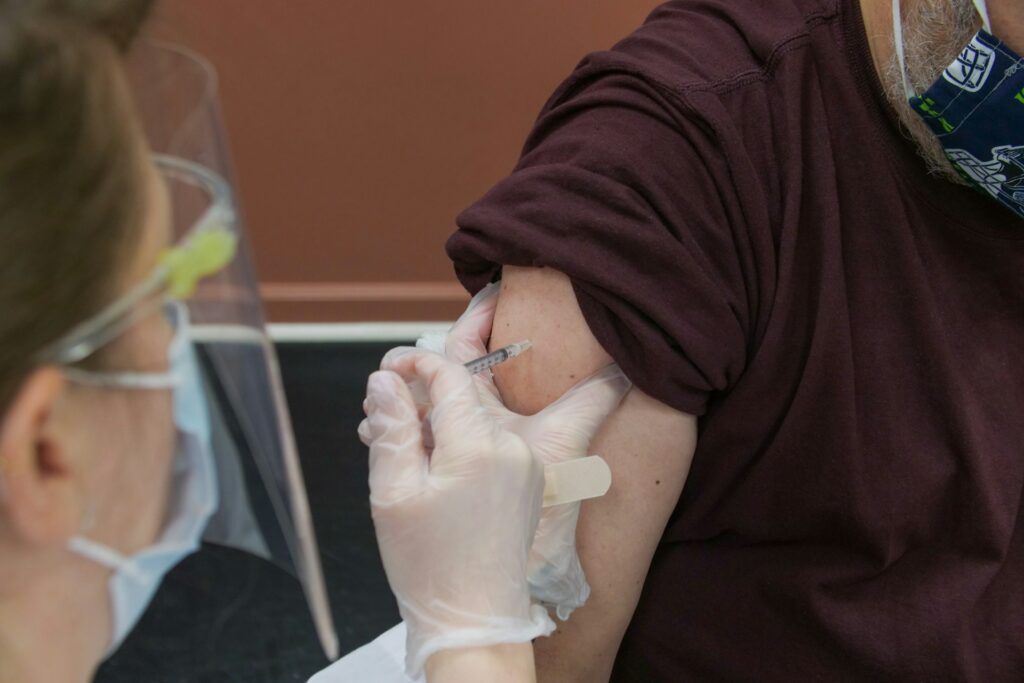
1. Vaccine challenges are front and center.
Vaccines continue to be one of the most contentious issues on the state level with over 150 vaccine-related bills already introduced in 33 states—a number that will undoubtedly increase.
So far, vaccine-related legislation can be broken down into a few categories:
Vaccine mandates and passports
There are a number of bills aimed at undermining state entities, employers, or businesses from mandating vaccines for employees, as well as undermining the ability to withhold services based on an individual’s vaccine status. This legislation is coupled with similar legislation aimed at prohibiting vaccine passports or prohibiting business or service providers from requiring proof of an individual’s vaccine status.
These bills, if successful, will undoubtedly throw a wrench in pandemic preparedness measures in the future. In addition, they could inhibit the ability of states to deal with new COVID waves, should they reach pandemic-era levels.
Vaccine exemptions
These bills cover a wide range of exemptions, from religious and philosophical exemptions to antibody exemptions for anyone who has tested positive for COVID can be exempt from a vaccine. (The latter falls apart the moment a new variant comes onto the scene.)
In Florida, one bill—with the support of Governor Ron DeSantis—aims to allow citizens to opt out of any medical intervention, including vaccines.
There is also a slew of bills targeting school entry requirements. While most bills are exclusive to COVID, vaccine opponents will likely attempt to extend them to other types of vaccines. Bills like these threaten to stress an already strained public health infrastructure and education system.
Human cell lines
As we have reported, human cell line legislation took aim at vaccines last year. We’re already seeing the projected continuation of that legislation in the early months of 2023.
As Rekha Lakshmanan, Chief Strategy Officer at The Immunization Partnership, said last year, “Many of us advocates are really, really worried that the decision is going to serve as a long-term catalyst and motivating factor for more of this kind of legislation to be filed. And to that point, what we also observed was a melding and consensus building amongst different fringe groups, in this case, the anti-vaxxers aligning with right-to-life organizations.”
Texas, for example, is presenting a bill on human cell labeling. This is not surprising, given we saw similar bills introduced in neighboring Louisiana last year.
Authority of health departments
Health departments were under tremendous scrutiny during the pandemic as they desperately tried to curb the spread of the pandemic and educate state residents about the need for vaccination against not just COVID but also flu and other vaccine-preventable diseases.
Bills are coming up that intend to erode the authority of the state’s Department of Health to declare a public health emergency and instead transfer that authority to state legislatures. Additionally, bills are being proposed to remove the health department’s authority to determine which immunizations are required for school entry, also with the goal of transferring that authority to the legislature.
One example of legislation being introduced in Texas weakens health department authority in determining school vaccine entry requirements.
Vaccine liability
Legislation aimed at holding employers, vaccine manufacturers, and state vaccine administrators liable (some criminally) for vaccine harm are emerging. Many are coupled with the proposal of state vaccine injury compensation funds, even though there are effective national programs such as the Vaccine Injury Compensation Program (VICP) already in place.
Examples of legislation being presented include one in North Dakota, which holds the department of health liable for any injuries from COVID vaccines.
Informed consent
These bills would require the healthcare professional administering vaccines—primarily targeting COVID vaccines but with potential to expand—to explain to the recipient all possible contraindications and potential harmful side effects. The healthcare professional would then be required to obtain written consent from the vaccine recipient. Such bills are meant to act as a deterrent to vaccines.
2. Medicaid coverage
With the recent attention on new Alzheimer’s therapies and the Food and Drug Administration’s (FDA) Accelerated Approval Pathway, there could be more attempts by state Medicaid programs to limit Medicaid access to therapies approved under this pathway.
Last year, for example, the Oregon Health Authority submited a 1115 waiver to Centers for Medicare and Medicaid Services (CMS) that would have restricted access to certain therapies through the state’s Medicaid program. The provision in the waiver dealing with Accelerated Approval was later removed by the Oregon Health Authority. While it is not known why it was removed, the removal came after the CMS requested a similar proposal be removed from the Tennessee TennCare III 1115 waiver. These types of provisions that attempt to restrict Medicaid access to prescription drugs often run afoul of federal statutes governing the Medicaid prescription drug program.
Additionally, with a recession looming and layoffs across a number of sectors, state legislators are preparing to tighten their belts. This could translate to restrictive budgets for state Medicaid funding, tax programs, and beyond.
But there is also good news on the Medicaid front. Oregon introduced a bill that aims to make much-needed changes to the rules governing the Oregon Health Authority’s Pharmacy and Therapeutics Committee and the Oregon Health Evidence Review Commission (HERC). Both of these entities are involved in determining how therapies are covered by Oregon’s Medicaid program.
“Coverage of life-saving therapies through state Medicaid programs is critically important for patients in Oregon and many other states,” said Patrick Plues, Vice President of State Government Affairs at the Biotechnology Innovation Organization (BIO).
3. PDABs aren’t going away.
Prescription Drug Affordability Boards (PDABs), which are state boards that aim to cap, control, and dictate the price of drugs manufacturers can charge, will continue to be actively introduced in 2023.
So far, legislatures in Minnesota, Virginia, and New Mexico have all already introduced bills to create prescription drug affordability boards. Such legislation may in turn have an impact on how Medicaid programs in those states provide coverage for therapies under PDAB review. Another issue is that state bills may use the federal Inflation Reduction Act’s (IRA) Medicare “Maximum Fair Price” (MFP) when setting an “upper payment limit” on a therapy.
“The MFP is simply another form of price control, and it could result in fewer therapies for patients,” said Plues. A recent study found that if the drug price controls included in the IRA had been in place over the last decade, just six of the 110 currently authorized medicines would have reached patients.
4. Workforce development will be a priority.
Though it is not yet clear how workforce development under the IRA will play out on the state level, it is a priority as states start to focus on how they will implement the bill. Additionally, workforce development in the biomanufacturing sector is a strong need as the U.S. and states still need to pick up the pace on pandemic preparedness nationally.
As BIO states in the 2021 Best Practices Report: “Because of current international supply chain challenges, there is a renewed emphasis on reshoring [important biomanufacturing] components of the innovation ecosystem, adding to the growing manufacturing presence of this segment of the industry in every U.S. state and territory.” As such, further assistance to biomanufacturing can act as a recession deterrent, as well.
Additionally, workforce development needs to encompass more than funds for high-level education and vocational training (though that is important). It also needs to incorporate short-term, focused training.
Between vaccines, Medicaid, PDABs, the IRA, and more, there is no doubt that it will be a busy year in state policy.